Brains, Gains And Growing Pains: Inside Mount Sinai’s BCI Crystal Ball
🍿Forbes Recap + "BCI State of Mind" video!
A few weeks ago I visited New York City to attend NYBCI25. Over three days I explored public perceptions about brain-computer interfaces, sponsored and co-hosted a mixer, and led a panel on BCI investing. My dear friend and longtime collaborator Woody Roseland joined me to bring the trip to life with his creative sensibilities and an industry outsider’s eyes. My Forbes recap follows. Enjoy!
🧠BCI State of Mind🗽
Brains, Gains And Growing Pains: Inside Mount Sinai’s BCI Crystal Ball
Originally published in Forbes, October 17, 2025
In their second annual Brain-Computer Interface Symposium, Mount Sinai Biodesign entreated attendees to an inside track on the top priorities and polarities defining BCI’s incomparable rise around the world.
The world’s leading companies, neurosurgeons, clinical researchers, investors, advocates, and other leaders past, present and future convened for two days at the New York Academy of Medicine. After last year’s inaugural gathering, this installment drew a full house of 440 attendees and over 50 speakers.
The program is the brainchild of Dr. Joshua Bederson, whose chairmanship of the neurosurgery department over the past 15 years has catalyzed Mount Sinai’s ascendance to a leading, “living” innovation hub for BCI and other breakthrough treatments. His opening remarks outlined a vision for full-spectrum discussion with the field’s leading voices. The myriad panels, heated debates, heartwarming stories, and innumerable conversations and networking came together to create a forum bustling with the intangible promises of discovery.
Protocol Labs anchored this year’s sponsorship, signaling accelerating private interests in supporting neural research platforms, alongside Precision Neuroscience, Synchron and other companies. BCI defies easy alignment, categorization, or containment, representing a shared challenge and opportunity. For a fecund field tasked with defining, refining, regulating and selling itself while accelerating in every direction in real-time, growing pains are inevitable.
The sessions included extensive presentations and discussions on technical advances and clinical methods, but the highlighted themes below are filtered through my lens of bringing this technology to people safely, sustainably, and responsibly, for all parties involved.
Minimal or More Invasive? Yes.

This chicken-egg neurotech question knows no better stoa than this event, which kicked off with a lightning panel of eight leading BCI neurosurgeons. Their nuanced and wide ranging conversation underscored that progress depends upon and demands a healthy tension between today’s products and tomorrow’s science. Or, was it today’s science and tomorrow’s products?
Invasiveness should not remain a limiting issue in BCI development, argued Michael Lawton, who leads Neuralink implantations as President and CEO of Barrow Neurological Institute. Lawton pointed to minimal scarring and chronic inflammation in both animal and human recipients of Neuralink’s n1 implant, arguing that the field should be aiming for “whole-brain BCI” as the goal, to read more data and develop more sophisticated applications.
Today, this goal represents a collective effort with many hands on deck. Paradromics shared updates of their benchmarking work to gauge information transfer in neural recording with their Connexus BCI. Blackrock’s long-awaited neural lace is still in development. Emerging companies like Axoft, Neurobionics and Neurosoft are advancing next-generation arrays.
In a later session, Ashwini Sharan, Medtronic’s chief medical officer of neuromodulation defended the medtech giant’s “right to compete” in the BCI space with adaptive DBS platform, cleared earlier this year, whose real-time read/write capabilities offer emerging evidence of the practical and clinical benefits of invasive tools, albeit for a narrow band of eligible patients.
As for minimalist BCI, the question of electing brain surgery looms large. Elad Levy, from the University at Buffalo, offered a pragmatic view of helping as many people today with the safest technology available in market. He likened today’s BCI to the Wright brothers airplanes, guaranteed to evolve in the next five years and beyond. Levy, who led the IDE study for Synchron’s minimally invasive Stentrode device, has devoted his career to advancing less invasive, less expensive, more accessible approaches to next-generation stroke treatment.
New neuroimaging tools are enabling the shift. As BCI propagate into neurosurgical suites around the world, precise brain mapping is emerging as a central need, pointed out Matthew Wilsey, a neurosurgeon at the University of Michigan who recently implanted Paradromics’ first human user. Levy outlined his use of Neuronetics’ Trakstar platform along with Medtronic tools, which have helped move implantation towards a faster, more affordable outpatient setting without compromising safety or quality.
Iahn Cajigas from the University of Pennsylvania has led implants of Precision’s Layer 7 array. He reviewed how the limits of fMRI and MEG are opening the door for more advanced “connectomics” to create “wiring diagrams” to guide neurosurgery. In a later session, Michael Ivan from the University of Miami shared how he has used Omniscient’s mapping tools to optimize placement and performance of Neuralink’s device as part of their ongoing clinical trial.
Show Vs Tell: Clinical Benefits
Chad Bouton of Northwell Health and founder of Neuvotion stole the show by revealing a “mind body connection” wherein Keith, a man living with a spinal cord injury whose double neural bypass was featured at this event last year, controlled a forearm device worn by Kathy, whose stroke left her partially paralyzed. Bouton explained, “When she holds something, he’s literally helping her close his hand, because she’s also living with paralysis. And he can now feel what she’s holding.”
In a video shared with attendees, Kathy sipped her diet coke, aided through over-the-skin wearables controlled by Keith’s thoughts. “It feels like you’re getting back a little bit of what was taken from you,” she told the audience, next to Keith. Neuvotion’s FDA-cleared sleeve is the first of a planned “neurorecovery ecosystem” of off-the shelf, BCI-compatible devices that defy easy value propositions in the best possible ways.
In a later session, Chris Kellner from Mount Sinai charted the rapid advances in stroke rehabilitation over the years, from breakthroughs in vagus nerve stimulation (VNS) systems, EEG-based wearable BCI, and both old and new attempts with cortical implants. Stroke impacts 800,000 new people each year, widening the berth of chronic patients who can benefit from specialized care. Kellner’s work shows that even patients with several years “between stroke and rehabilitation can still have a robust improvement” using standard scales of functional assessment.
Quantifying clinical benefits of BCI, whether in stroke, ALS, spinal cord injury, or other areas, remains the central challenge for commercial companies approaching regulatory clearance and reimbursement decisions. Jamie Brannigan from Mount Sinai encouraged conservative market estimates and tight clinical definitions for suitable BCI recipients. Omid Forouzan, VP of clinical affairs at Synchron, was among several leaders who highlighted the progress and pitfalls of advancing objective measures of digital autonomy, contextual functional independence, activities of daily living considered critical or “instrumental,” and other ways to measure value to end-users.
Mariska Vansteensel from UMC Utrecht Brain Center translated these tactical quality of life measures (feels, functions, survives) into the overarching concept of relational personhood, which gauges how people are, and feel, perceived as full persons by each other. Perhaps Congress needs a bit of the Dutch touch.
Video Games and Blame Games
In the first hour of the event, Lawton shared a video showing Brad Smith, one of Neuralink’s trial participants, playing games with his children through his BCI.
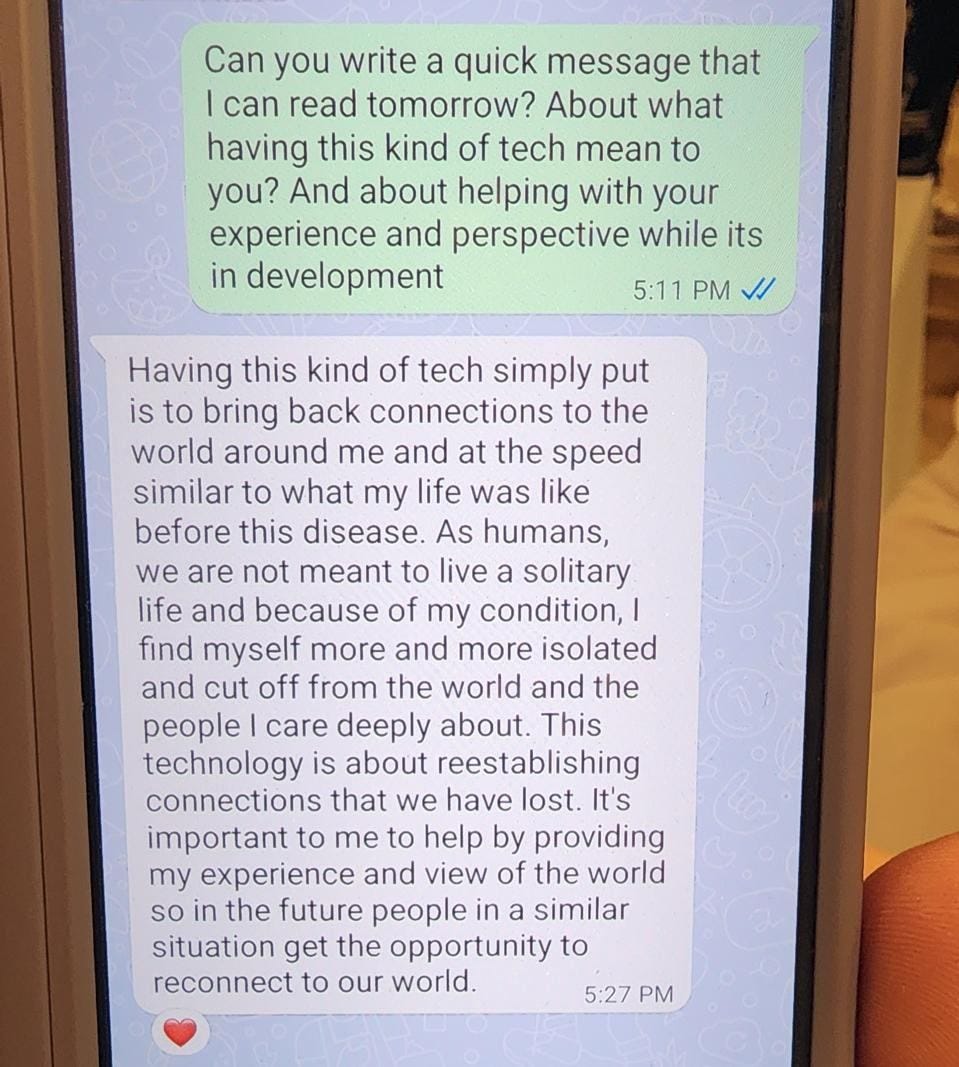
In the next session, when Ian Burkhart, founder of the BCI Pioneers Coalition, asked ALS Caregiver and patient advocate MA Fernandez about how BCI could help her family, she answered “I really want Jules to be able to play videogames with Skyler,” referring to her husband and eight-year old son.
“[BCI’s value] is about establishing that connection between those two brains – one who is growing through a life altering event that’s generating so much wisdom, the other who is developing as a child. The only thing that my husband has is his capacity to think about the things he wants to do. Creating a bridge between that and the world, particularly for us as a family, is something I want to see in this world. But, video games are not on a list of things the FDA or payers are looking for.” - MA Fernandez, ALS Caregiver & Patient Advocate
Indeed. Former CMS Medical Officer Lee A. Fleischer opened the very next panel by saying “it’s very clear that video games don’t fit the reasonable, necessary criteria” after explaining that “CMS cannot pay for things unless Congress says so.” Fleischer pointed to past approvals for speech generation and exoskeleton devices, encouraging innovators to “understand the process and work within the structures because if not, you don’t win.” But when he said the agency’s job is to consider only the benefit and not the cost of new technologies, his panelists pushed back.
Mijail Serruya from Thomas Jefferson University called out “quite a bit of hypocrisy in what is paid for” by CMS, cautioning that one-size fits all reimbursement in BCI “could be a trap” because people with identical impairments often have different functionality. David Putrino from Mount Sinai argued passionately that at the bedside, his job was to help drowning people, only for insurers to deny coverage by countering “people are not drowning, their legs just aren’t working. Send us more documentation on the legs.”
“We’re in a new age,” Putrino declared. “Technology is advancing faster than ever before, and if we need to use the same system of checks and balances to get treatments through, it will die on the vine before it can help people. My very big fear about BCI is that if it flops now, it’s going to be 20 years before there’s any investor confidence to move forward.”
Valleys of Death and Silicon
Later that afternoon I moderated a panel with four neurotech investors to discuss market trends, commercial strategies, funding growth and evolution, categories versus platforms, globalization, and founder/funder dynamics.
My panelists included Sean Escola, Head of Neurotech and NeuroAI at Protocol Labs, Peter Zhegin, Neurotech Director at e184, Adam Caplan, Founder at Jumpspace Ventures, and Konstantinos Alataris, BOD at Precision Neuroscience, Managing Director at Forepont, and former founder and CEO of Nevro.

This year’s funding for implanted BCI will double or triple last year’s $500+ million, driven by Neuralink’s recent $625 Series E. But beyond growth, the key investment trend to watch is the diversification of capital, traditional VC, strategic philanthropies, cryptocurrency firms, international government programs, and beyond. Zhegin observed this is not a purely market-driven trend, but one stemming from investor interest and personality factors. Escola described braiding grant dollars and venture dollars to optimize support across pre-commercial development.
Alataris wants to see new market access options for BCI that diverge from traditional med tech commercialization paths. His remarks followed a series of case studies he’d presented earlier to illustrate how the lag between FDA approval and CMS reimbursement restricts new product commercialization for both large and small med tech companies.
This so-called valley of death hinders innovation and limits exit options for startups: “You spend 10 years getting to FDA approval, a minimum of $100 to 150 million, then you need to raise another $300 million to create your sales force, and you live and die based on this single product revenue. But there is no commercial strategy that can work with no robust reimbursement. So aligning FDA approval and reimbursement is key.”
All speakers advised founders navigating this landscape to understand their company’s value fully, and take time to explore all dimensions of the strategic value conferred by different types of backers on clinical and operational goals, from hiring to raising the next round. In a later question about founder X-factors in BCI, they mentioned the ability to secure both grant and venture dollars, quickly launch a research lab, along with requisite technical expertise and capabilities across the founding team.
Alataris called on founders to opt for strategic capital and partnerships in big tech over med tech, while exploring profitable paths to sharing technology, revenue, IP, and maybe even data, with other companies, with an eye on building multiple product applications. In discussing BCI platforms, he compared the potential to the smartphone ecosystem and app store, Zhegin envisioned developer ecosystems like those at Intel or NVIDIA, while Caplan named read and write capabilities demonstrating sustained safety and performance. Escola called for more modular infrastructure across the ecosystem, so that each BCI company won’t have to absorb millions in equipment costs, and can focus more on business innovation.
Science x Economics = Ecosystem
Building economies of scale in BCI will require vision, intention, and action.
Science Corp’s Founder and CEO Max Hodak returned to this year’s event to share a heady list of updates that spanned bringing their retinal prosthesis closer to market, inventing synthetic proteins to enable optogenetic biohybrid arrays, and launching a new, next-generation technology stack to “rewrite the economics of BCI development.” Science’s enablement toolkit includes neural probes, next-generation headstage devices, and a full back end of software applications.
Hodak said this modular approach “cuts the total development costs of bringing BCI therapy to market from over fifty to less than five million dollars.” Coming from a single company, these developments demonstrate the platform approach discussed earlier.
Similarly motivated offerings to scale innovation through interdependence are underway from Mint Neuro and Neuvotion, while new tools like Omniscient’s platform could also smooth the path for BCI startups. While these market innovations represent potential boons for a higher throughput R&D flywheel in the near-term, the longer term impacts on commercial BCI companies’ costs, value proposition (and value perception), and pricing strategies remain open questions.
From the outside, a successful BCI ecosystem requires external leadership and vision, too. Beyond attracting diverse pools of capital as described above, neurotechnology’s momentum has drawn interest from public leaders outside the US federal government. Viq Pervaaz, the Senior Vice President of Life Sciences & Healthcare at NYCEDC, shared a macro-economic lens on skilled job creation, catalyzing company formation, leveraging healthcare and life sciences anchors, socioeconomic impact, and more.
NYEDC’s ongoing partnership with Mount Sinai has also supported development of new cutting-edge treatments, entrepreneurship and spinouts, and deeper synergies in development. At a time of political gridlock and federal clawbacks across health, science, and global leadership, NYEDC and Mount Sinai’s system-driven collaboration offers a relevant playbook for leaders seeking to future-proof their economies.
Consumer Taxonomies and Economies
To build an application layer, BCI needs an application taxonomy, a mindset shift that has already brought the field to a crossroads. Bloomberg reporter Ike Swetlitz opened his panel of seven BCI executives and neurosurgeons by asking for perceptions about Neuralink’s claimed goals to implant “otherwise healthy people” in five years.

Several panelists including Tom Oxley, Founder and CEO of Synchron hedged their answers with disclaimers about tight FDA definitions, Musk’s exuberance and the collective need to prioritize safety over rabbitholes. To be fair, Synchron’s sedulous leadership in advancing metrics and standards could arguably benefit this field even more than their high-profile partnerships with Apple and NVIDIA.
And yet, the blitz of progress in the last year alone is publicly documented and undeniable: BCI is coming. Neuralink has gone from zero to 12 patients with permanent implants in the US and Canada in under two years. Precision has secured an FDA clearance, and in the time it took to publish findings from their first five patients, increased users by more than tenfold to 50+ people.
In a notable breakthrough, Precision’s cofounder and chief science officer Ben Rapoport shared that this now includes patients without disabilities, such as a woman being treated for tumor removal who opted into the temporary implant to help advance research. “Building functional maps from healthy individuals could yield detailed maps of hand, speech and sensory representations,” said Rapoport, adding that such maps could also inform BCI implant targeting.
Beyond the lane of motor neuroprostheses, some panelists pushed back on the definition of “healthy people,” pointing to differences between everyone, as well as market shifts towards a broader audiences and elective same-day procedures. Paradromics’ founder and CEO Matt Angle spoke openly about his dual vision as a “precisely defined medical device,” as well as a platform technology to turn capital-intensive R&D into “applications that unlock a broader future.” Jacob Robinson, founder and CEO of Motif Neurotech and author of the application taxonomy paper in question spoke of the BCI field’s shared goal to “create technology that is more normalized, with capabilities designed to bring us to the present.”
His comments, underscored by Helen Mayberg later in the program, stressed that psychiatry suffers from need for more quantifiable, objective assessments. “It’s important to understand what’s happening in the circuit that drives your patient’s suffering. We’re not used to seeing dramatic, fast changes, in people who have failed to respond to years of medication.”
Mayberg, a neurologist and director at Mount Sinai, pointed to 20 years of promising evidence of DBS’s efficacy in treating people living with severe depression. She urged the importance of closing clinical gaps with neurotechnology, starting with the lack of validated “facial or behavioral measures, or better, a measure from the brain, that tells us if they’re on the right track or need an adjustment.”
Mayburg’s closing observation was that “these patients don’t know who they used to be. We’re having to chase the disorder by defining the disorder.” It reminded me of Alan Watts’s line, which applies to people as well as the BCI field as a whole: “Trying to define yourself is like trying to bite your own teeth.” Teeth gnashing about slow diagnostic innovation aside, BCI is evolving towards broader applications that can fit under the category of “human problems.”
Ignacio Saez from Mount Sinai stressed that unlike motor BCI development, for cognitive and psychiatric BCI, “scientific knowledge, and our understanding of the neurobiological basis of cognitive and psychiatric processes, is still a major limiting factor.” Unrelated to implanted BCI, Meta shared in a separate session that their newly launched EMG wristband took 6,000 training sessions for off the shelf-functionality, suggesting that we are in the early innings of this next frontier.
Michael Ivan, Neuralink’s PI at the University of Miami, said our modern understanding of the brain “is that multiple areas work together to create something powerful. Together, they shape executive function, attention span, memory. As a neurosurgeon, I try to avoid these areas. But with BCI it’s the opposite – we’re trying to find these functional areas to figure out how to sense from them and stimulate them.”
Where Do We Go From Here?
With their second event, Mount Sinai’s BCI Symposium has cemented itself as a must-attend for those interested in the nexus of brain health and implanted technologies. With an eye on returning to New York in 2026, where I will hope for more interdisciplinary and diversified panels, as well as representation from newer companies in BCI and beyond, some closing thoughts on what’s ahead.
Lab to Living Room: Beyond “bench to bedside,” BCI research needs to prioritize open, curious exploration of what happens inside a patient’s home. Jen French mentioned FDA’s CDRH director Michelle Tarver’s remarks from a recent iBCI-CC meeting, calling for a shift in care models to meet people at home, with “integrative person-centered solutions that move care into the environments where people live, work, and play.”
Stick to the Plan: BCI Godfather Leigh Hochberg offered a time capsule of his “Top 10 characteristics of the Ideal Neural Interface/BCI” from 2006: Safe, consistent, autonomous, real-time control, no added concentration, works at speed of speech or typing, aesthetically acceptable, reliable, affordable, and available. The only thing I’d add for today’s AI era is data autonomy.
Maintain Cooperation: Nobody expects anything but more advances from Neuralink, which received a glowing outlook from Morgan Stanley the week after this event. But despite surging interest and positive momentum, one executive I spoke with shared his fears of industry cannibalization. From competing for the same venture dollars, talent, market indications, differential pricing strategy, and winning public mindshare, BCI companies are only going to grow more competitive. But ongoing solidarity will help the industry gain traction where and when it matters most.
Anticipate Federal Challenges: Dire government dysfunction looks to continue, impacting strategy for regulatory submissions, non-dilutive funding, coverage decisions, as well as the ability to engage with new programs like WISeR and active legislation like the newly proposed MIND Act. This bill aims to “confront the mental privacy gap” by restricting federal contracts to neurotech companies who fail to meet a checklist that the FTC must pull together in the next year.
The right kind of healthcare-ification: Daniel Rubin, from Harvard Medical School and Massachusetts General Hospital, shared MGH’s launch of the country’s first BCI Clinic, which aims to be a clinical center of excellence that coordinates care across specialties and technologies, starting before diagnosis and continuing through device implantation and ongoing care management. Beyond benefiting patients, families, and clinical teams, such a unified model could facilitate cost-effectiveness studies that could prove valuable to the industry in the coming years.
Lastly, I asked MA Fernandez if I could share the message her husband Jules shared with attendees. As a young father to an 8 year old son, his ALS symptoms began six years ago, and have progressed to the point of his being confined to a wheelchair, dependent on a feeding tube and a breathing tube. Their story broke my heart but gave me hope.
How much longer will it take before we can turn to BCI to help folks like Jules?
Originally published in Forbes, October 17, 2025






Hey, great read as always. Mindful Pilates gains new depht.